Making History: My Experience in the Moderna COVID-19 Vaccine Trial
February 20, 2021
It was Sunday, February 7. My parents got a call that I had been accepted into the Moderna TeenCove vaccine trial, a program to ensure that the Coronavirus vaccine is safe for adolescents ages 12-17. I was ecstatic to be part of history. From the phone call, I was scheduled to have my first out of four appointments, which took place on Wednesday, February 10. Over the next few days, I had trouble containing my excitement.
When Wednesday came, I got up early in order to get to the appointment in Warwick, Rhode Island. I have to say that I was a bit nervous since I didn’t know how the appointment would go and what it would entail. Once I arrived, I went to the clinical trial office, where I was met by an intake nurse who would be one of many professionals that would see me. While taking a thorough medical history, she reiterated how safe the trial was. Hearing such confidence from an expert helped calm my nerves about the study.
Once the medical history was completed, a medical assistant came in to take my vital signs and helped me download an app on which I would complete daily surveys. On the app, I was also told my unique identification number, which described my place in the study. Next, the study doctor came in quickly to check my heart and lungs. Once she was done, a phlebotomist came in to take my blood and a COVID-19 nasal swab, all of which was conducted without pain or complications.
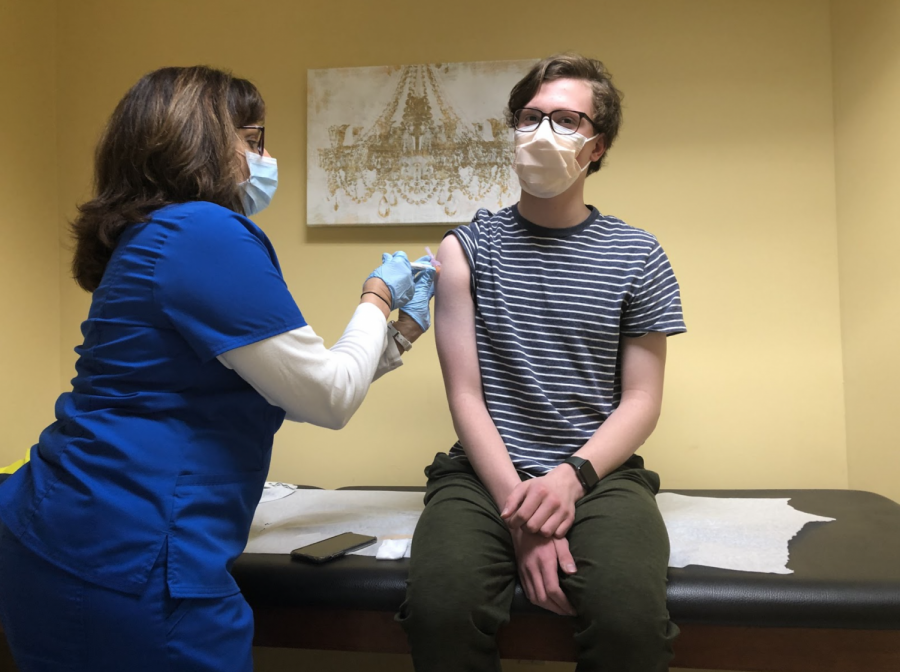
After about fifteen minutes of waiting, the big moment came: I was finally going to be injected with either the vaccine or the placebo. The vaccinating nurse came in with the unmarked syringe, which had been dyed brown in order to hide whether or not I received the vaccine. Following the injection, I had to wait around for half an hour in order to see if I had an allergic reaction. Thankfully, I experienced no symptoms other than a pounding headache and sore throat.
Following the wait, the medical assistant came again and informed me that my first series of surveys were available on the app. For the surveys, I had to take my temperature using a particular thermometer I was given, as well as record my symptoms, similar to the wellness check we complete every day before school. Afterwards, I was able to check out and say goodbye to my caregivers until my second injection.
It felt extraordinarily special and important to be part of a study that will change the course of history. It feels triumphant that I am working to make a difference in the world by ensuring the safety and efficacy of this vaccine. I will receive the second dose on March 15 and have between two and four appointments afterwards, where I will be evaluated and have more labs drawn. Now, I don’t know whether or not I received the vaccine or not, which concerns me, but I do know that my participation in the trial is helping kids across the globe.